Confidential Reporting System for Surgery (CORESS)
“Safety in Surgery Symposium”
Introduction by Chairman – Lord Ribeiro CBE FRCS, Past President Royal College of Surgeons of England
3rd June 2021 | 14:00 – 16:30

Confidential Reporting System for Surgery (CORESS)
“Safety in Surgery Symposium”
Introduction by Chairman – Lord Ribeiro CBE FRCS, Past President Royal College of Surgeons of England
3rd June 2021 | 14:00 – 16:30
00:00 Lord Ribeiro’s introduction and welcome
3:41 Dr Kevin Stewart
36:09 Professor Frank Smith, CORESS
43:00 Professor Tom Clutton-Brock
1:12:48 Mr Jerard Ross
1:50:45 Professor Peter Brennan
Lord Ribeiro
Gave a short introduction, paying tribute to the speakers and topics who have attracted an event registration of 148+.
Dr Kevin Stewart, HSiB
Provided a background to HSIB talking about the ethos, to learn from other industries and bring the processes into healthcare, providing recommendations are to system level bodies.
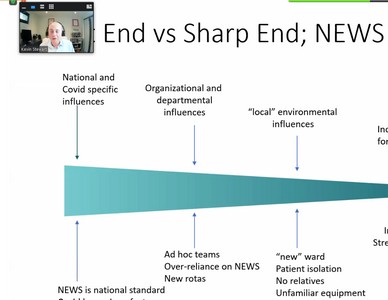

Dr Stewart’s talk focussed on a recent report on Never Events, published in January 2021 which was an analysis of HSIB’s national investigations. The report looked at 10 past events in detail in order to assess whether they really were ‘Never Events’. The report concluded that many were not Never Events as they did not fit the definition.
Dr Stewart received questions:
Question: How items are taken to HSIB and how vetted? Answer: There is a form on their website, HSIB are keen to get reports from people at the sharp end.
Question: Will HSIB be involved in the maternity programme? Answer: HSIB focus is elsewhere at present.
Question: Is the introduction of the role of Director of Patient Safety a useful one, this role could work with organisations like CORESS? Answer: HSIB are supportive of this approach but limited in their ability to lobby at present.
Professor Frank Smith, CORESS
Provided an overview of CORESS, provided direction to the website explaining the charity set up the event and thanking the four speakers who are representing their own safety organisations. CORESS website details were provided.
Professor Thomas Clutton-Brock, Medical Devices Testing and Evaluation Centre
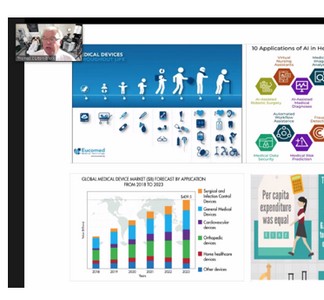
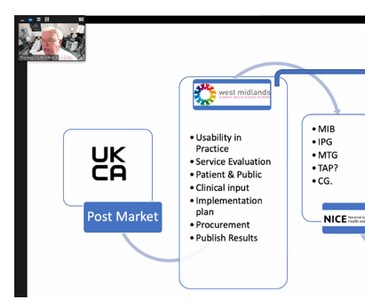
Professor Clutton-Brock talked about medical devices, the expense in relation to these and covered what falls into this category, for example software. He talked about regulation, implementation, cost, the difficulty of bringing new technology to the point of use and what the future looks like. He also covered COVID and the UK manufacturing response for ventilators leading on to training and usability testing. Professor Clutton-Brock concluded that failure to innovate is a patient safety issue and walked through the process of getting technology through approval.
Question: Quality assurance of equipment sent to the UK, how is this ensured? Answer: Regulations, however while these can be met but the product can still be shoddy.
Questions: How AI might be used e.g. its observance of surgeons to alert them to potential errors? Answer: AI benefits have a wealth of potential e.g. recording the movements of skilled surgery or for imaging, there is however a need to consider how to adopt it safely.
Mr Jerard Ross, MDU

Mr Ross provided an overview of MDU and ran through the scenario of a clinical incident and how, potentially, the clinician becomes the second victim of an incident. He identified the multiple strands the clinician may encounter from a civil claim, NHS complaints procedure, CQC inspection through to media for example. He provided guidance on how a clinician can approach the procedure positively and protect themselves.
Professor Peter Brennan, Chair of Council BOAMS


Professor Brennan outlined the level of human error in everyday life and the importance of focussing on yourself first in order to effectively look after your patient; taking breaks, eating regularly, remaining hydrated, being aware of emotional charge and the impact of tiredness. He covered team work, empowerment, communication and situational awareness. Professor Brennan finished with lowering hierarchy and the benefit of challenge without retribution.